The root chain must be numbered from the end nearest a double bond carbon atom if the double bond is in the center of the chain the nearest substituent rule is used to determine the end where numbering.
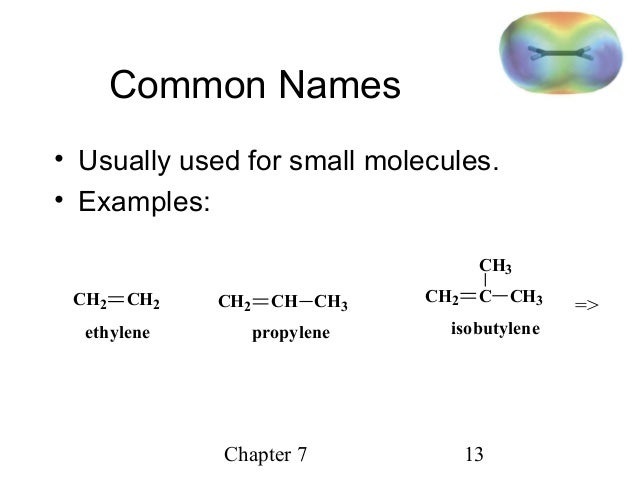
Common name of alkene vinyl.
An industrially important example is vinyl chloride precursor to pvc a plastic.
Alkenes can be straight chain branched chain or cyclic.
Draw the kekulé condensed or shorthand structure of an alkene cyclic or acyclic given its iupac name.
The term is often used as synonym of olefin that is any hydrocarbon containing one or more double bonds.
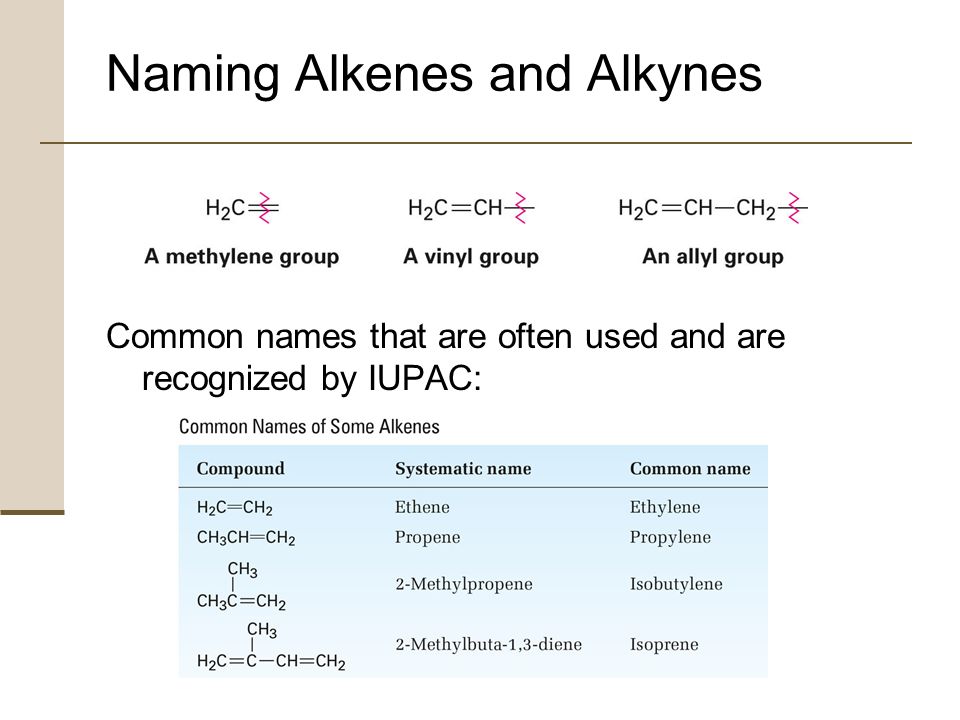
That you should know are vinyl substituent h 2 c ch allyl substituent h 2 c ch ch 2 allene molecule h 2 c c ch 2.
By arthur winter.
The longest chain chosen for the root name must include both carbon atoms of the double bond.
There are a couple of unique ones like ethenyl s common name is vinyl and 2 propenyl s common name is allyl.
Pyridine is a basic heterocyclic organic compound with the chemical formula c 5 h 5 n it is structurally related to benzene with one methine group ch replaced by a nitrogen atom.
It is a highly flammable weakly alkaline water miscible liquid with a distinctive unpleasant fish like smell pyridine is colorless but older or impure samples can appear yellow.
The chain is to be numbered from the end that gives the lowest number to the carbon in double bond.
That you should know are.
Give the iupac equivalent of the following trivial names.
Remove the ane suffix and add ylene.
Substituents are to be numbered according to their positions in the chain and listed alphabetically.
A three carbon alkene is named propene and an alkene in a five membered ring is named cyclopentene as shown here.
In chemistry an alkene is a hydrocarbon that contains a carbon carbon double bond.
Iupac rules for alkene nomenclature.
There are a couple of unique ones like ethenyl s common name is vinyl and 2 propenyl s common name is allyl.
Alkadiene alkatriene etc or polyene for acyclic hydrocarbons with two or more double.
The general formula for alkenes with one double bond is c n h 2 n.
In chemistry vinyl or ethenyl abbreviated as vi is the functional group with the formula c h ch 2 it is the ethylene iupac ethene molecule h 2 c ch 2 with one fewer hydrogen atom.
Alkenes have a carbon to carbon double bond.
Simple alkenes often have common names but all alkenes can be named by the system of the international union of pure and applied chemistry and have the ending ene.
Remove the ane suffix and add ylene.
The name is also used for any compound containing that group namely r ch ch 2 where r is any other group of atoms.
Whereas the names of alkanes end with the suffix ane alkenes end with the suffix ene a two carbon alkene therefore is named ethene.
If you know how to name alkanes adding alkene nomenclature to your repertoire is a fairly straightforward task.
However the iupac recommends using the name alkene only for acyclic hydrocarbons with just one double bond.
Draw the structure of a vinyl ethenyl and allyl 2 propenyl group and use these names in alkene nomenclature.
Vinyl substituent h 2 c ch allyl substituent h 2 c ch ch 2 allene molecule h 2 c c ch 2.